Electrochemical AFM (EC-AFM)
Electrochemical atomic force microscopy mode allows for AFM measurements while the electrochemical reaction is taking place. The AFM itself is not part of the electrochemical reaction, it only acts as an observer. In particular the AFM tip is not conducting and on floating potential to ensure it does not interfere with the electrochemical reaction. With a special electrochemical cell changes on the electrode surface can be monitored in an electrolyte solution. Both the reduction reactions at the cathode and the oxidation reactions at the anode can be studied. Knowledge of these reactions is crucial in applications such as corrosion, batteries and photovoltaics. Thus EC-AFM allows in-situ monitoring of the electrode structure during such reactions. As a result, the relation between the electrode structure / morphology and its electrochemical activity can be established.
Electrochemical studies are usually done in fairly aggressive liquid environments. Thus excellent environmental control and protection of AFM electronics is necessary for good imaging results. The Nanosurf Flex-Axiom combined with the EC-AFM sample holder is ideally suited for these measurements. All the AFM electronics is protected and positioned above the liquid. Special corrosion-resistant material is used for the cantilever holders.
Shown below is a study of the nucleation and growth of copper clusters under electrochemical control. Cyclic voltammograms were used to charactize the electrochemical reactions and derive suitable potentials to observe the topographical changes under stable electrochemical conditions.
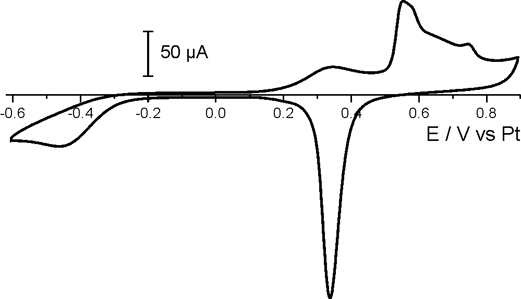
Nucleation was observed by AFM at E = -50mV in sulphoric acid vs. a platinum quasi reference electrode. Increasing the potential to E = -10mV reduced the growth rate and a single copper cluster could be followed by time-lapse AFM. This reaction is reversible and at positive potentials the cluster was dissolved again.
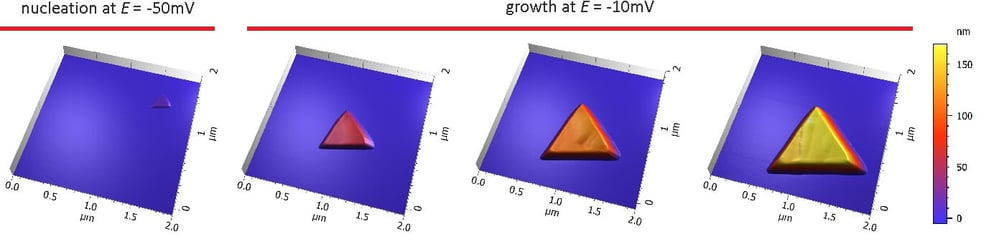
Data courtesy: Ilya Pobelov, University of Berne, Switzerland
The height of the ECS204 electrochemical stage is designed so that it can also accommodate standard, rod-like electrodes. Further it is equipped with a sealing membrane and gas in- and outlets to allow oxygen free conditions and also means to exchange the electrolyte. In the example below, the dissolution of deposited copper clusters on a polished rod-like platinum electrode at oxidizing conditions was observed by AFM.