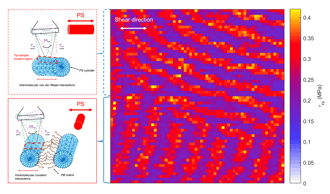
Subsurface nanomechanical properties using torsinal force spectroscopy.
01.09.2025
The ultimate tool for nanoscale research from biological molecules to advanced new materials.
The versatile mid-range research AFM that grows with your demands in modes and accessories.
A compact affordable research AFM that is astoundingly easy to use, with more than 30 modes and options.
Fastest reliable sub-Angstrom surface roughness metrology.
Bringing the power of DriveAFM to a wafer metrology system purpose-built for the requirements of the semiconductor industry.
Measure roughness and other material properties of heavy and large samples up to 300 mm and 45 kg.
For unique requirements, we will design a bespoke AFM solution, leveraging our decades of engineering expertise.
Slide an AFM onto your upright optical microscope turret for a leap in resolution.
One of the smallest ever AFMs, created for integration into custom stages or existing setups.
A flexibly mountable research-grade scan head for integration into custom stages or existing set ups.
What is atomic force microscopy (AFM)? How does AFM work? What AFM modes do I really need? How do I get started with AFM?
Learn how AFM works with cantilever/tip assembly interacting with the sample. Explore CleanDrive technology, calibration methods, and feedback principles for precise nanoscale imaging.
An overview of common AFM modes. To learn about each mode in more detail and see application, view the full article.
We regularly publish detailed reviews providing practical guidance and theoretical background on various AFM applications.
Read detailed technical descriptions about selected AFM techniques and learn how to perform specific measurements on Nanosurf instruments.
A library of links to research papers in which Nanosurf instruments were used.
Learn AFM from our library of recorded webinars, covering different measurement techniques, modes, and areas of application.
Short video clips explaining how to perform different operations on Nanosurf instruments.
Watch a product demonstration to learn about the capabilities of our AFMs.
Short videos of our AFMs.
Browse news articles, press releases and a variety of other articles all around Nanosurf
Browse Héctor Corte-Léon's weekly experiments, for inspiration, entertainment, and to discover everyday applications of AFM.

Héctor here, your AFM expert at Nanosurf calling out for people to share their Friday afternoon experiments. Do you know how long glass can last? You will learn:
A few weeks ago I was in Rome, and while I was doing some tourist stuff and visiting museums I came across a few examples of Roman glass. Imagine my surprise: in my mind, humanity didn't adopt the use of glass until centuries later, and yet, there it was. This piqued my curiosity, when was glass first manufactured, and why there aren't there more examples of glass objects in museums?
I mean, glass tends to be fragile, and the longer something exists, the more likely it becomes to get destroyed... but still, if glass was commonly used for centuries, there should be far more examples, so what is going on? Does glass age? If so, maybe I can see that ageing using an AFM.
Not all glass is made the same.
First thing to understand is that glass has changed a lot through the centuries. We improved its formulation and manufacture to make it withstand higher forces, hotter temperatures, and highly acid/basic environments.
The basic component of glass is silica (see Ref 1).

Silica tends to form crystals.

The problem is that these crystals are extremely brittle, and break very easily, also, it takes a huge amount of time for perfect crystals to grow, so usually silica tends to form glass instead of crystal.

However, even in glass form, silica alone tends to make very brittle glass. To make silica glass stronger impurities are added. This is the so called 'soda-lime-silica' or 'potash-lime-silica' glass, and it is a mixture of silica (SiO2), the alkali oxides soda (Na2O) or potash (K2O), and alkaline earth oxides such as lime (CaO). The ratio between silica and the other components varies the melting point, being the general rule more silica means higher melting point.

Soda-lime glass means an improvement compared to regular silica glass, however, these glasses tend to break when heated, and degrade relatively easily in acid environments. To compensate for this, Borosilicate glass was created (see Ref 2). The use of Boron improved glass properties massively, in particular, borosilicate glass can be heated and used in cooking or chemical reactions (commercial names for this type of glass are: Borosil, Duran, Pyrex, Glassco, Supertek, Suprax, Simax, Bellco, Marinex, Heatex, Endural, Schott, Refmex, Kimax...).
 This doesn't cover all glasses, but as a general description should suffice.
This doesn't cover all glasses, but as a general description should suffice.
Now, what else is there to know?
Glass ages.
Glass ages in two very obvious ways, it can break because of an impact, or it can get scratched because something abrasive was pressed and slid against its surface.
It also ages in several non obvious ways (non obvious because it usually take centuries, see Refs 3, 4, and 7).
When glass is in contact with a solution, it develops something called a hydration layer.
 The hydration layer is the basis of the leaching process.
The hydration layer is the basis of the leaching process.
The hydration layer is where the solution molecules start entering the glass. The solution molecules then tend to free the Na and Ca ions. This creates a layer of weakly coupled silica particles called the gel layer. By the way, did you know that the thickness of the hydration layer can be used in archeology to date glass? See Ref 3 if you want to know more.

Over time, the Na and Ca ions tend to float away while the Silica particles tend to accumulate around the places where the ions where removed (i.e. where the particles break from initially).
This process continues making the pits deeper and the craters larger... until the hydration layer can no longer reach more ions.

This part of the leaching ageing process can be natural or artificial. For instance, in Borosilicate glass, this process is induced by putting the glass on an acid environment so it develops a natural layer of silica particles than prevent the glass from degrading further. However, what happens with other types of glass where the process can continue further?
In soda-lime glasses the leaching process can dig deeper pits. This can then develop into two scenarios. In the first one, commonly called cloudy glass, the surface of glass becomes so amorphous that the glass is no longer transparent. This is similar to having millions of tiny scratches all over the glass surface.
 Cloudy glass image taken from Kai Hendry
Cloudy glass image taken from Kai Hendry
In the second scenario, the pits go deeper into the glass and develop cracks, this is called crizzling. Depending on the size and distribution of the cracks, the glass might or might not lose its transparency, but it certainly becomes more brittle.

Crizzled glass examples adapted from Ref 6.
Is this all? No.
There is yet another scenario where the solution is rich in ions and silica, and instead of eroding the surface of glass, new layers are deposited. This is usually referred to as patina formation.

Curiosity fact. The patina sometimes can create such uniform layers that they produce light interference effects (i.e. the glass shows different colors that change according to the light's angle). This not only affects the appearance of old glass, but also has very interesting photonic properties (see Ref 8).
 Patina formation example adapted from Ref 8.
Patina formation example adapted from Ref 8.
By the way, a very serious side-effect of glass leaching has to do with papyrus conservation. It seems papyrus are regularly conserved in between glass panels in many museums around the world... well, what people doing restorations noticed is that over time papyrus develops white patinas. Those residues are formed from the silica and ions released by the glass panels used to encapsulate the papyrus (see Ref 5). In some cases this made the papyrus unreadable, and potentially damaged forever.
In the AFM lab.
In order to see if I can detect glass degradation with the AFM, I chose glass pieces that are easily available to everyone. I chose a Borosilicate microscope glass slide to test if Borosilicate is indeed resistant to leaching, I took a champagne flute (I remember that when I was a kid these were easily damaged by dishwasher machines), a glass candle holder from a very famous Swedish furniture manufacturer, and a decorative Frederich vase popular in the 60s (and hence very likely made of soda-lime glass).

The first step was to determine what was going to be my degradation procedure to simulate the passage of centuries in a few hours.
At first I tried ageing glass by putting it into the dishwasher, but after 50+ washes, I couldn't see any degradation on the champagne flute glass... so I turned to more aggressive methods.
So I look at the Ph of common products in the house and found that vinegar and lemon juice are the most acidic substances and drain cleaner the most basic... but after soaking glass in all of these substance for a few days... I saw no degradation.
It was time to look into chemistry.
I found two things, one that if I want to remove Na and Ca ions I need an acid like HNO3, and second, that red colored glass like the one in the Frederich vase are likely made of metals that are affected by Nitric acid (Au in bulk is not dissolved by nitric acid, but Au ions are likely to be formed, and then deposited again on the surface of glass) .

Once I selected Nitric acid as the ageing agent (this will be similar to letting the glass in soft water, just x times faster), I needed a cleaning agent, for which I choose Hydrogen Peroxide (H2O2). The reason for this is that I tried it in the past to clean glass slides with nice results (do you remember that fridayAFM? https://www.nanosurf.com/fridayafm/fridayafm-can-we-see-changes-in-glass-wetability ). I will also use a lens cleaning tissue to dry the glass afterwards.
So... first test, see if the cleaning and ageing affects the Borosilicate microscope slide. To capture these images I used WaveMode imaging with a WM08 probe. The setpoint was 1 - 2 nN (larger than that I noticed that probes could get damaged and become blunted), the WaveMode frequency was 5 - 10 kHz, and the free vibration amplitude about 5 nm peak to peak. Line rate was between 0.5 and 1 Hz.

First thing to notice is that the surface of the borosilicate glass is indeed full of volcano-like pits like the ones described in the degradation process to passivate the surface. Second thing to notice is that these structures do not change after the cleaning or after being in acid for 12h. So far, this is what we expect, the borosilicate glass being stable.
Next, lets see the candlestick holder. I used the same imaging parameters, but I have to confess that I got three holders because I didn't knew in which condition they will be stored or manipulated, but after a few tries in different areas and cleaning, I came up to consistent and reproducible results:

To my surprise, it seems the surface was already treated with acid. Maybe, as we mentioned before, this was done to create a passivation layer of silica particles that will prevent the hydration layer to reach deeper into the glass. Proof of this is that the acid treatment doesn't seem to affect the pits.
The big particles that we see when zooming out are likely Ca crystals. This could be native or residues from cleaning with water with high Ca concentration. The fact that they leave almost no pitting when gone (purple arrows and bottom image after the acid treatment), makes me believe they are not part of the surface but deposited there because of the cleaning the manufacturer does (if it was my cleaning, we will see it in the other samples also).
Next was the champagne flute.

This one surprised me a lot, because the surface didn't show pitting, but it was not flat either, in some areas it almost resembled pitting... but not quite. Until I zoomed out and I saw the big hole and the "smaller" pits around. This made me suspect that the champagne flute might have a protective coating, so I put it in acid for another 12h. The result as you can see is that the coating degraded and revealed the layer underneath. Just by looking at the thickness of this deposited layer, I think it might be a patina layer, formed by dipping the flute into acid with high silica content, or it could be a completely different coating aimed at protecting the glass against acidic drinks and the dishwasher.
Last but not least, the Frederich glass, the one that is most likely soda-lime glass. If we are going to see some ageing, it will be here.
 At the beginning it looks like the glass is covered in contamination and I might not be able to properly clean it, less see any pitting or ageing effect. But after the first dip in acid, there they were the volcano-like pits. So I let it for another 12h in acid, and this really did the magic, the contamination was mostly gone, and the surface was full of pits. Hence this is likely soda-lime glass, and this is glass ageing that we can see thanks to the extreme sensitivity of AFM.
At the beginning it looks like the glass is covered in contamination and I might not be able to properly clean it, less see any pitting or ageing effect. But after the first dip in acid, there they were the volcano-like pits. So I let it for another 12h in acid, and this really did the magic, the contamination was mostly gone, and the surface was full of pits. Hence this is likely soda-lime glass, and this is glass ageing that we can see thanks to the extreme sensitivity of AFM.
Let's recap. Glass ageing is an ubiquitous problem which might not happen in one week, or even in one year, but it is there damaging glass all around the world. So far it was only possible to study it in detail in archeological samples, but thanks to instruments such as Nanosurf's DriveAFM, it is possible to investigate this phenomena in reasonable time scales. Here I used several types of glass (microscope slides, champagne flutes, candlestick holders and fancy vases) to show how simulated ageing (using Nitric Acid) affects each type of glass. This revealed that the borosilicate glass was mostly untouched by the acid, the acid-treated candlestick holder also resisted pretty well, but the coated flute saw its coating degraded, and the Frederich vase saw pitting appearing.
I hope you find this useful, entertaining, and try it yourselves. Please let me know if you use some of this, and as usual, if you have suggestions or requests, don't hesitate to contact me.
If you are interested in further details on how to clean the samples and how to setup the AFM, here is the video with those details.
Oh, I almost forgot, the oldest known man-made glass is 34 centuries old (see wiki article).
One of the first records of glass, appears in the Egyptian faience, which is a type of coating with glass-like properties.
 No
No
Egyptian faience ushabti of Lady Sati. New Kingdom, Dynasty XVIII, reign of Amenhotep III, c. 1390–1352 BC. Possibly from Saqqara. Source: https://en.wikipedia.org/wiki/Egyptian_faience
References:
[1] https://www.glassflake.com/blog/2021/07/05/molecular-engineering-perfecting-glass-composition/
[2] https://en.wikipedia.org/wiki/Borosilicate_glass
[3] Lanford WA. Glass hydration: a method of dating glass objects. Science. 1977 May 27;196(4293):975-6. DOI 10.1126/science.196.4293.975.
[4] Zanini, R., Franceschin, G., Cattaruzza, E. et al. A review of glass corrosion: the unique contribution of studying ancient glass to validate glass alteration models. npj Mater Degrad 7, 38 (2023). https://doi.org/10.1038/s41529-023-00355-4
[5] Graf, Jörg, Villmann, Beate and Schlattner, Evelyn. "Composition and source of white precipitations on the inner side of papyrus glazings " Restaurator. International Journal for the Preservation of Library and Archival Material, vol. 39, no. 2, 2018, pp. 85-107. https://doi.org/10.1515/res-2018-0002
[6] Majérus, O., Lehuédé, P., Biron, I. et al. Glass alteration in atmospheric conditions: crossing perspectives from cultural heritage, glass industry, and nuclear waste management. npj Mater Degrad 4, 27 (2020). https://doi.org/10.1038/s41529-020-00130-9
[7] Gin, S., Delaye, JM., Angeli, F. et al. Aqueous alteration of silicate glass: state of knowledge and perspectives. npj Mater Degrad 5, 42 (2021). https://doi.org/10.1038/s41529-021-00190-5
[8] Giulia Guidetti, et al. Photonic crystals built by time in ancient Roman glass. Proceedings of the National Academy of Sciences, Vol 120, https://doi.org/10.1073/pnas.2311583120

01.09.2025
Subsurface nanomechanical properties using torsinal force spectroscopy.
01.09.2025
How to calibrate nanomechanical measurements

12.06.2024
Students from Kirschgarten Gymnasium explore AFM technology at Nanosurf, gaining practical insights into nanotechnology ...

08.12.2024
Learn how to make a Python code to interface your AFM with a gamepad.

01.10.2024
FridayAFM: learn how the extreme sensitivity of AFM can reveal the glass ageing process.
11.07.2024
FridayAFM: learn how to perform datamining on large sets of AFM data.
Interested in learning more? If you have any questions, please reach out to us, and speak to an AFM expert.
